 Acuity Brands President & CEO Neil Ashe: “None of us know what demand is going to look like for the next three, six, nine, 12 months. So we’re positioning ourselves to attempt to be successful in whatever market environment presents itself to us.”
Acuity Brands President & CEO Neil Ashe: “None of us know what demand is going to look like for the next three, six, nine, 12 months. So we’re positioning ourselves to attempt to be successful in whatever market environment presents itself to us.”
Your humble editor listened in on the Acuity Brands investor call yesterday and there was some pretty good information on how Acuity Brands is faring during the COVID-19 pandemic. The results were better than Wall Street expected and the stocked jumped 7% yesterday on the numbers. Below are my key takeaways from the discussion:
- Acuity is adding talent and beginning to deploy teams focused on executing our digital transformation.
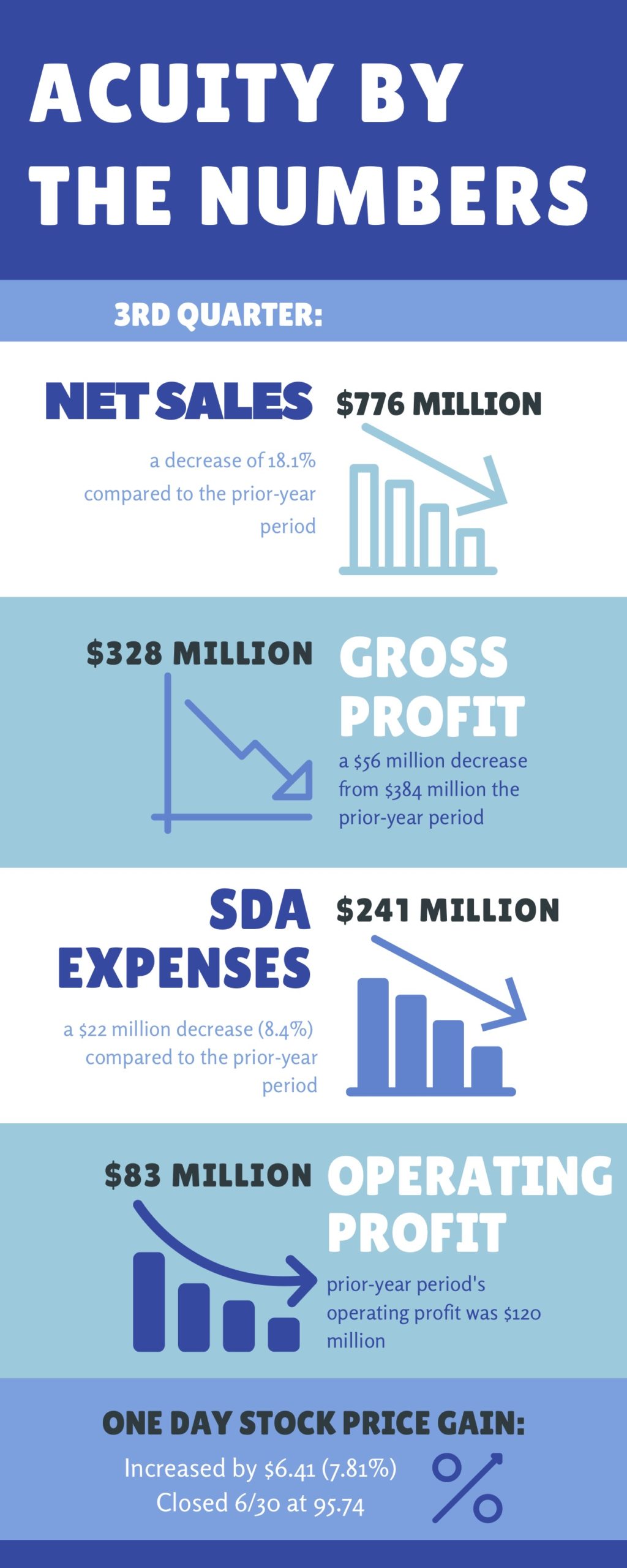
- Performance this quarter demonstrates the adaptability and durability of the business. While revenue declined 18%, largely due to the COVID-19 pandemic, gross profit margins grew 170 basis points and generated $150 million of free cash flow during the quarter.
- Net sales through the independent sales network, which makes up approximately 75% of total net sales, was down approximately 10% over the prior year.
- Direct Net sales were down 31% over the prior year third quarter, due primarily to weakness in large projects that have been postponed due to COVID, as well as some large projects in the year ago period that did not repeat this year.
- Corporate Account Net sales were down 59% this quarter compared with the year ago period, due primarily to the impact of COVID on retail customers.
- SG&A expenses decreased approximately $22 million compared to the year ago period. The decrease in SG&A expense was primarily due to cost in response to the lower sales, including freight, commissions, travel expenses and marketing costs and the benefits of streamlining activities, partially offset by the addition of costs from acquired businesses.
- Indoor positioning and heat mapping technology is used to keep shoppers safe through social distancing measures in grocery stores and pharmacies.
- Ushio has agreed to supply Acuity Brands with its Care222 UV disinfection module, which uses filtered excimer lamps to generate 220-nanometer far-UVC light capable of inactivating viruses and bacteria on indoor surfaces. The agreement is exclusive to Acuity Brands for general illumination uses throughout North America. The benefit of 222 besides being very effective in clinical testing is that, it’s not harmful to the skin and the eyes of humans. The 254, which is in the market today and then there’s 405, that’s been around for quite a while. The 405 is not as effective against viruses and then the 254 is harmful to the skin and eyes.
- Pricing pressure is increasing. Last year Acuity led the industry well in price increases in response to tariffs. This year, pricing is down a bit.
- Acuity believes they clearly took orders from competitors who were unable to ship. Acuity believes competitors will try and respond to the downturn by lowering price to try and gain share.
- In June, virtually all of the construction sites opened back up early in the month and started seeing a pickup in the demand for stock and flow as distributors feel more confident. The job sites are open and need to stock inventory and replenish inventory that may have been used throughout.
- The Education Market is taking advantage of the schools being empty to go forward to the extent that they have funds to go ahead and take advantage of cost saving renovation opportunity, energy-saving opportunities.
- Retail has been in a big slowdown, but retail is very interested in the UV for obvious reasons.
- Infrastructure is beginning to come back again. The backlogs for most of the agents are solid. Distributors are seeing a pickup in the stock and flow.
- New construction has been slower to come back.
Toward the end of the Q&A, CEO and President Neil Ashe stated, “None of us know what demand is going to look like for the next three, six, nine, 12 months. So we’re positioning ourselves to attempt to be successful in whatever market environment presents itself to us.”





